GMP for Cannabis
03.04.2019

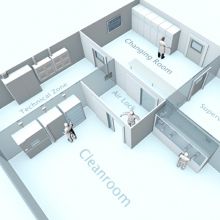
As reported in INCB´s 2018 Report, more and more countries allow cannabis for medicinal purposes. In March 2017, the national German legislature expanded the options for prescribing medical cannabis products by passing a law amending provisions under the Narcotics Law and other regulations. These Cannabis products, however, must comply with the relevant requirements laid down under Medicinal Law, including GMP and GDP, and in addition, laid down under Narcotics Law. Therefore, the BfArM (the German Federal Institute for Drugs and Medical Devices) has taken over new responsibilities by establishing the Cannabis Agency. This agency is meant to help in ensuring supplies for medical-quality cannabis.
Unlike AGES in Austria, though, where cultivation of medical cannabis has already been established, cannabis will not be cultivated by the BfArM itself, but by commissioned companies. Cannabis is not meant to be stored directly at BfArM during any stage of the purchasing, harvesting or distribution process. These steps will be carried out by relevant producers or other commissioned companies (i.e. suppliers, importers). Hence, the agency will manage and monitor the cultivation, harvest, processing, quality assurance, storage, packaging and distribution of cannabis to wholesalers, chemists or manufacturers. The GMP inspectorates are responsible for issuing manufacturing and import licenses or GMP Certificates. Thus, they will perform inspections at the sites of manufacturers who apply for these certificates and licenses.
Source: www.gmp-compliance.org